What is the Solvent Redistribution (SR) method?
The solvent redistribution (SR) method is based on observing the redistribution of the solvent in response to an external stimulus such as the addition of solutes, changes in pH, temperature or pressure. The solvent is not simply a background but an active medium that responds to the smallest amounts of external stimulus.
Solvent Redistribution method as applied to solubility and aggregation
Solubility is about the solute dissolving in the solvent. Its not only about the solute but also about the solvent molecules around the solute! The SR method utilizes how the solvent redistributes around the solute molecules (it can be solid, liquid or gas) to obtain information about the aggregation state of the solute clusters. Here we show the redistribution of the solvent at different stages of aggregation:
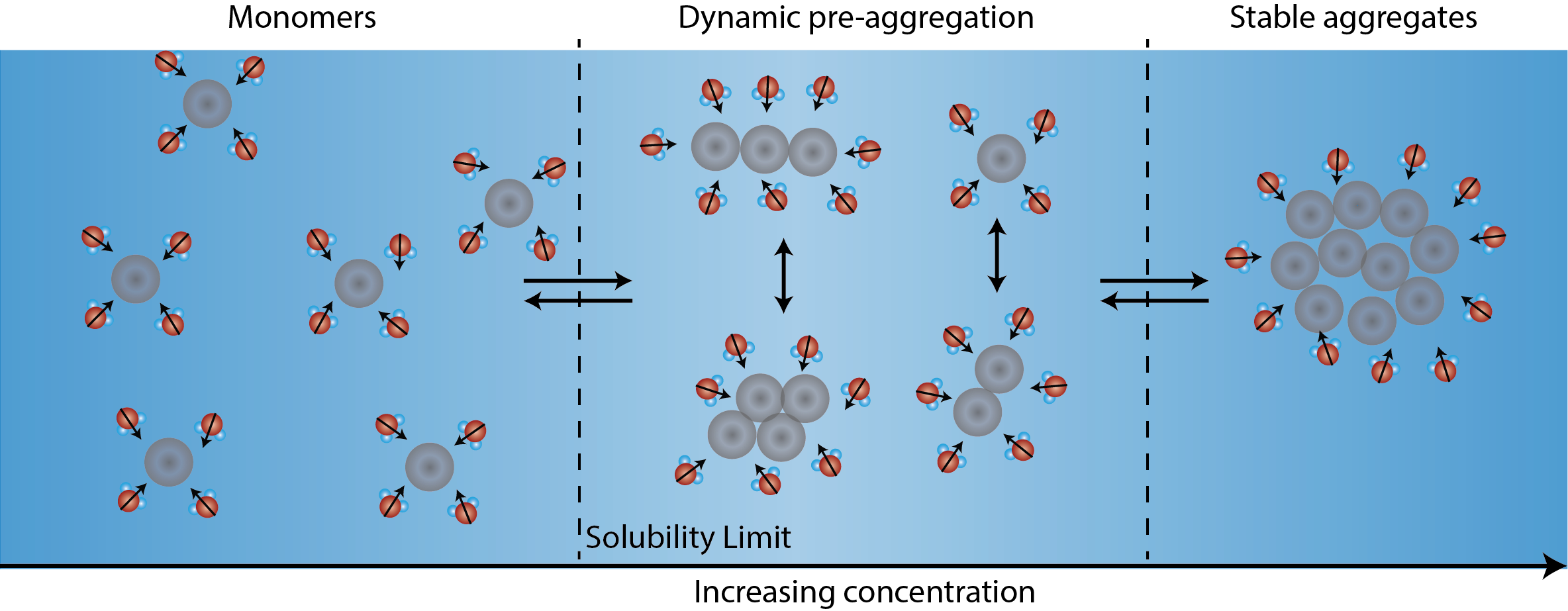
Following the different stages of aggregation using the Solvent Redistribution method. With increasing concentration of solute in solution, solute monomers transition to stable aggregates. In-between fully dissolved monomers and stable aggregates, there is a concentration range where different types of solute structures (dimers, trimers, etc.) co-exist. Correspondingly, the solvent molecules around these dynamic structures redistribute and fluctuate. The technology behind the Solvent Redistribution method is called second harmonic scattering that directly measures the solvent molecules around the solute structures. At the onset of aggregation (the transition from monomers to dimers, trimers), the number of solvent molecules around the solute structures increases. This increase in interfacial solvent molecules lead to a steep increase in the second harmonic scattering intensity, which is used as a metric to measure the solubility limit.
How does the SR method change the landscape of solubility measurements?
The SR method is introducing a fundamental change on how solubility and aggregation is measured. While typical methods focus on observing the solute, the SR method shifts its focus on the solvent itself, which is an active medium. Small additions of solute is felt as a whole by the solvent molecules, making it sensitive to small additions (nanomolar concentrations) of solute. The solvent redistribution method is not limited to solubility measurements. It is also applicable to measurements of aggregation, crystallization and stability of substances. It has a wide application coverage, from different types of samples to different types of solvents. The only condition is that the solvent must be dipolar (e.g., water or any aqueous-based biologically relevant media, DMSO, methanol, ethanol and so forth). The solutes can be small molecules, fragments, proteins and macromolecules.
The SR method, as a light scattering technique is rapid and sensitive and requires minimum amount of compounds (read our Application Note here). It uses the solvent that is native and fundamental to the chemistry of solubility and aggregation. The SR method does not require the use of filters, filter-plates, vials, columns, and minimizes the use of chemicals and consumables, a sustainable and economical method for solubility and aggregation measurements.
